Contact:
4008465777
Tonghua Dongbao's Entire Insulin Lineup Secures Category A in the VBP Program, With Two Types Categorized as A1
On April 23, 2024, China's Joint Procurement Office (JPO) announced the preliminarily selected bids from a continued round of the national volume-based procurement (VBP) program for insulin. This VBP cycle is set to run from the date of tender implementation until December 31, 2027.
In the latest round of the insulin VBP program, Tonghua Dongbao Pharmaceutical Co., Ltd. (hereafter referred to as "Tonghua Dongbao" or "the Company") successfully secured a Category A designation for its entire lineup of insulin products. Notably, the Company's insulin analogs, Insulin Glargine and Premixed Insulin Aspart (Aspart 30 injection and Aspart 50 injection), were both categorized as A1. These results meet management expectations and align with the Company's development planning. Benefiting from its diverse product portfolio and economies of scale, Tonghua Dongbao is poised to further increase its market share and sustain a steady stream of reasonable profits following this successful bid.
Unlike the initial round of the insulin VBP program, this round notably included Tonghua Dongbao's new flagship products, Premixed Insulin Aspart 30 and 50 injections, for the first time in the procurement process. Their designation as Category A1 is expected to significantly accelerate nationwide access to and distribution of these products. The Company now offers a comprehensive portfolio of rapid-acting, basal, and premixed insulin products, encompassing both human insulin and insulin analogs, all of which have been recognized as Category A/A1 in the VBP program.
The VBP selected bids from Tonghua Dongbao include the full range and specifications of Human Insulin Injection, Insulin Glargine Injection, as well as rapid-acting Insulin Aspart and the Premixed Insulin Aspart 30 and 50 injections. The table below details the prices and the contracted purchase volumes agreed upon for each product.
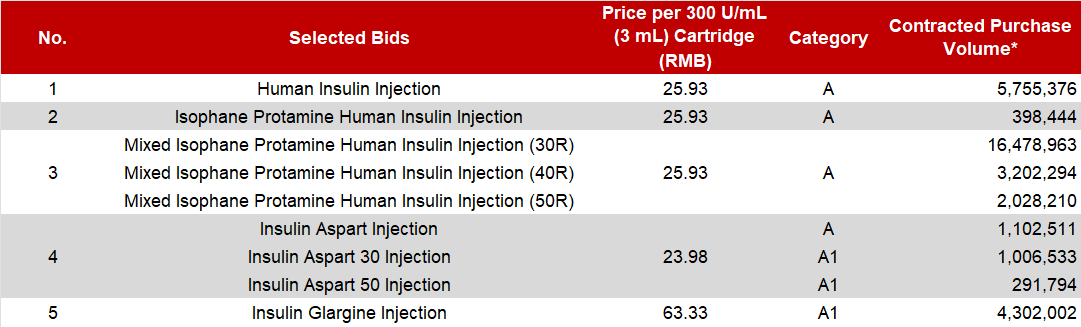
* Note: The prices, categories, and purchase volumes of the aforementioned products are subject to the final data released by the JPO.
In addition to the contracted purchase volumes, the selected products will also be entitled to a portion of the remaining volumes chosen by medical institutions within the same procurement group. This includes the unallocated volumes demanded for Category B and C products and non-selected products, collectively amounting to over 33 million vials. This potential addition in purchase volume is expected to boost the Company's market share and help offset the adverse impacts of price reductions on its performance. The Company will capitalize on this VBP round to further expand the sales of its insulin products, particularly insulin analogs, expedite market entry, and increase market share.
Mr. Li Jiahong, Chairperson of Tonghua Dongbao, remarked, "As a leading insulin manufacturer in China, Tonghua Dongbao prioritizes the health and interests of patients. In this round of VBP, we proactively responded to the National Healthcare Security Administration's call for the VBP of pharmaceuticals. All our products were designated as Category A. Our key insulin analogs, Insulin Glargine and Premixed Insulin Aspart, won the bids with the lowest prices within their respective groups. Tonghua Dongbao fulfills its corporate social responsibility by lowering drug prices to help reduce relevant out-of-pocket costs for people covered by medical insurances, lighten the financial burden on patients, and enhance drug accessibility and affordability."
Besides its strong foothold in the insulin market, Tonghua Dongbao remains steadfast in driving innovation and transformation in the treatment of diabetes and other endocrine disorders, with intensified efforts in novel drug development. This commitment enables the Company to continually expand its range of therapeutic categories while broadening the indications for its products. For gout drugs, the Phase IIa clinical trial of the first-in-class URAT1 inhibitor (THDBH130 tablets) reached its primary endpoint; the Phase I clinical trial of XO/URAT1 dual inhibitor (THDBH151 tablets) also met its primary endpoint and showed first-in-class potential. For GLP-1 novel drugs, two first-in-class antidiabetic drugs, namely the oral small-molecule GLP-1 receptor agonist (THDBH110 capsules) and the dual GLP-1/GIP receptor agonist (THDBH120 injection), completed the enrollment of the first participant in the Phase I clinical trial for diabetes indications. Moreover, the Center for Drug Evaluation, NMPA accepted the clinical trial application for weight loss indications of the dual GLP-1/GIP receptor agonist (THDBH120 injection). These milestones mark the significant progress Tonghua Dongbao has made in enhancing its R&D and operational efficiency and deepening its commitment to innovation.
The Company is also expanding its international presence, accelerating the strategy to globalize its products, and striving to create new growth engines. For insulin products, the Company's application for marketing authorization of Human Insulin injection was accepted by the European Medicines Agency. The Company also established a strategic partnership with Kingfriend to jointly enter the U.S. insulin market with three insulin variants: Aspart, Glargine, and Lispro. Furthermore, the Company expanded the registration and application of Insulin Glargine and Insulin Aspart in developing countries. For GLP-1 products, the Company collaborated with Kexing Biopharm to expedite the registration and application processes for Liraglutide in 17 emerging markets. To date, registration applications have been filed in several countries. With the expansion and diversification of its product pipelines and portfolio, the Company is bringing more high-quality products, including human insulin and insulin analogs, GLP-1RA, and first-in-class drugs, to the international market. This is part of its strategy to accelerate internationalization through various channels and means.
Looking ahead, Tonghua Dongbao will utilize this round of VBP to boost domestic sales and market share of its insulin analog products. Meanwhile, the Company will further its commitment to innovation and transformation by enhancing its R&D capabilities and actively seeking partnerships. This two-pronged strategy of "Internal R&D and External Cooperation" will broaden the Company's product portfolio. The Company will also step up its global presence to introduce more high-quality products to international markets, driving sustainable growth in sales.










